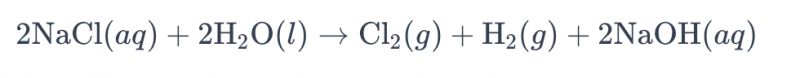Usoro nke electrolyzing a brine ngwọta iji titanium electrodes na-emepụta chlorine a na-akpọkarị "electrolysis nke brine." N'ime usoro a, a na-eji electrodes titanium arụ ọrụ iji kwado mmeghachi omume oxidation nke ion chloride na brine, na-eduga na ọgbọ nke chlorine gas. Ngụkọta kemịkalụ n'ozuzu maka mmeghachi omume bụ nke a:
Na nha nha a, ion chloride na-enweta oxidation na anode, na-ebute mmepụta nke gas chlorine, ebe mkpụrụ ndụ mmiri na-ebelata na cathode, na-emepụta hydrogen gas. Ọzọkwa, a na-ebelata ion hydroxide na anode, na-eme hydrogen gas na sodium hydroxide.
Nhọrọ nke electrodes titanium bụ n'ihi nguzogide na conductivity nke titanium dị mma, na-enye ya ohere ịme mmeghachi omume ahụ nke ọma n'oge electrolysis na-enweghị corrosion. Nke a na-eme ka titanium electrodes bụrụ ezigbo nhọrọ maka electrolysis nke brine.
Electrolysis nke mmiri saline na-achọkarị isi iyi ike mpụga iji nye ike maka mmeghachi omume electrolytic. Isi iyi ike a na-abụkarị ọkụ ọkụ ọkụ (DC) n'ihi na mmeghachi omume electrolytic na-eme ka ntụzịaka na-aga n'ihu na-aga n'ihu ugbu a, na ọkụ ọkụ DC nwere ike ịnapụta ntụziaka dị ugbu a mgbe niile.
N'ime usoro ịmegharị mmiri saline electrolyza iji mepụta gas chlorine, a na-ejikarị ọkụ ọkụ DC dị ala na-arụ ọrụ. Voltaji nke ọkọnọ ike na-adabere na ọnọdụ mmeghachi omume kpọmkwem yana imewe akụrụngwa, mana n'ozuzu ya na-adị n'etiti 2 ruo 4 volts. Na mgbakwunye, ike ọkụ ugbu a bụ oke dị oke mkpa nke kwesịrị ikpebi dabere na ọnụ ụlọ mmeghachi omume yana mkpụrụ mmepụta achọrọ.
Na nchịkọta, nhọrọ nke ọkụ ọkụ maka electrolysis nke mmiri saline na-adabere na ihe ndị a chọrọ nke nyocha ma ọ bụ usoro mmepụta ihe iji hụ na mmeghachi omume dị mma na ịnweta ngwaahịa ndị a chọrọ.
Oge nzipu: Jan-16-2024